Infection Prevention, Sterilization, and Green Cleaning
What are healthcare associated infections?
What does OSHA mandate with regard to worker safety?
How does healthcare differentiate between general surface cleaning, disinfection, & sterilization?
What are some environmentally preferred techniques for general surface cleaning?
What are some environmentally preferred techniques for sterilization?
 Overview
Overview

The topics of infection prevention, disinfection, sterilization, and green cleaning in healthcare can be confusing. With the rise of “superbugs” such as Clostridium difficile (C. difficile), Methicillin-resistant Staphylococcus aureus (MRSA) and their related healthcare-associated infections (also known as HAIs), the need to evaluate infection prevention strategies has never been more critical. Information about lowering toxicity in healthcare facilities may become entangled with the requirements to keep facilities as disinfected and infection-free as possible. Though MnTAP advocates for the reduction of hazardous materials whenever possible, we also acknowledge the complexities and trade-offs that Minnesota healthcare facilities face. Please note that information on the Centers for Disease Control (CDC) Healthcare-Associated Infections website always supersedes the information on our website.
What are healthcare associated infections?
As defined by the Centers for Disease Control (CDC), health care-associated infections (HAIs) are infections that people acquire while in a healthcare setting, as they are receiving treatment for another condition. HAIs can be acquired anywhere, including inpatient hospitals, outpatient settings, and long-term care facilities such as nursing homes and rehabilitation centers. HAIs may be caused by any infectious agent, including bacteria, fungi, and viruses, as well as other less common types of pathogens. One of the dangers of HAIs is that many are evolving to be multi-drug resistant organisms (MDROs), meaning they are difficult for providers to treat and can result in serious illness or even death of the patient. This illness or death is independent of the symptoms or diagnosis that brought the patient to the facility in the first place.
HAIs are associated with a variety of risk factors, including:
- Use of indwelling devices such as bloodstream and urinary catheters
- Surgical procedures or injections
- Contamination of the health care environment
- Transmission of communicable diseases between patients and healthcare workers
- Overuse or improper use of antibiotics
The CDC has produced several free training videos that may aid you or your staff in better understanding HAIs and their associated risks. The best way to prevent HAIs is to have your staff work in close contact with an onsite (or consultative) Infection Control Practitioner (ICP). In addition, many organizations exist that can help you stay current on new developments in this field, including the Association for Professionals in Infection Control and Epidemiology (APIC), the National Institute of Allergy and Infectious Disease (NIAID), and the Organization for Safety, Asepsis and Prevention (OSAP) for the dental industry. The Joint Commission and the Society for Healthcare Epidemiology of America (SHEA) co-produced a book titled Best Practices in Infection Prevention and Control available for purchase on the Joint Commission’s (JC) website.
What Does OSHA Mandate with Regard to Worker Safety?
According to the Occupational Safety & Health Administration (OSHA), employers must ensure the greatest possible protection for employees in the workplace. OSHA maintains that cooperative efforts by both employers and employees will help establish and maintain a safe and healthful work environment. With regard to the healthcare sector, one way an employer must maintain a safe and healthful work environment includes preventing worker exposures to blood, body fluids, and infectious diseases by way of contact, droplet, or airborne transmission. More OSHA information on infectious diseases and how to protect healthcare workers can be found here. Information on bloodborne pathogens and infectious waste can also be found on MnTAP’s healthcare infectious waste page.

Your Employee Right-to-Know (ERTK) program must include:
- An inventory of hazardous substances and/or agents that exist in the workplace (this includes cleaning agents, disinfectants, and sterilants);
- Identification of employees who are routinely exposed to those substances or agents;
- A system for obtaining and maintaining written information about the substances and agents employees may be exposed to in the workplace;
- Methods for making ERTK information readily accessible to employees in their work areas;
- A plan for providing initial, pre-assignment and annual training of employees; and
- Implementation and maintenance of a labeling system or other warning method.
In addition, the following OSHA rules apply when using any chemicals in any workplace in the United States:
Employers are responsible for:
- Performing a “hazard assessment” of the workplace to identify and control physical and health hazards;
- Identifying and providing appropriate PPE (personal protective equipment) for employees;
- Training employees in the use and care of the PPE;
- Maintaining PPE, including replacing worn or damaged PPE; and
- Periodically reviewing, updating and evaluating the effectiveness of the PPE program.
Employees are responsible to:
- Properly wear PPE;
- Attend training sessions on PPE;
- Care for, clean and maintain PPE; and
- Inform a supervisor of the need to repair or replace PPE.
How Does Healthcare Differentiate Between General Surface Cleaning, Disinfection, and Sterilization?
Different levels of cleanliness are needed for different activities in healthcare. As a rule, we recommend using the lowest cleaning level that meets your needs and is accepted by your Infection Control Practitioner.
In general, there are three categories of equipment used in healthcare: noncritical, semi-critical, and critical items. They are defined by the CDC as follows:
- Noncritical items
Defined as those items that come in contact with intact skin but not mucous membranes (intact skin acts as an effective barrier to most microorganisms). As it relates to cleaning processes, low-level disinfectants can be used for items such as crutches, blood pressure cuffs, patient furniture, and computers. Intermediate-level disinfectants should be used for items that may be in contact with nonintact skin for a brief period of time (such as bed rails, bedside tables, hydrotherapy tanks, and others, as defined by your Infection Control Practitioner).
- Semi-critical items
Defined as those items which have the potential to come into contact with mucous membranes or nonintact skin. This includes items such as respiratory therapy and anesthesia equipment, some endoscopes, laryngoscope blades, esophageal manometry probes, cystoscopes, anorectal manometry catheters, and diaphragm fitting rings. These items must, at a minimum, be subject to a high-level disinfectant. Sometimes, a sterilant is most appropriate for certain semi-critical items. A consultation with your Infection Control Practitioner is best.
- Critical items
Defined as items that enter sterile tissue or the vascular system. This includes surgical instruments, cardiac and urinary catheters, implants, and ultrasound probes used in sterile body cavities. Purchasing already-sterile items or using steam sterilization is preferred for critical items. Sometimes, other sterilization techniques are recommended by the manufacturer.
There are also three types of cleaning. These types of cleaning should not be confused with noncritical, semi-critical, or critical items, which are categories of equipment.
- General Surface Cleaning
General surface cleaning physically removes all visible dirt, organic matter, and bacteria. It is normally accomplished with water, mechanical action like scrubbing, and detergents or enzymatic products. Surface cleaning should always precede disinfection and sterilization. If organic matter is not first removed, it can inactivate disinfectants. In many locations throughout a healthcare facility or clinic, general surface cleaning is the highest level of cleaning necessary, such as in waiting rooms, hallways, nursing stations, and so on. There are several ways to achieve a “green cleaning” program, and suggestions on environmental best practices are offered in the next section. You should consult your Infection Control Practitioner to determine what can be “surface cleaned only” in your facility. - Disinfection
Disinfection reduces the risk of infection from microbial contamination. All disinfectants used in a healthcare setting must be on the EPA‘s list of registered antimicrobial products. Probably the best guidelines available on disinfection come from the CDC (last updated in 2008) and are relevant for many types of healthcare facilities. The chemical disinfectants discussed in the CDC guidelines include alcohols, glutaraldehyde, formaldehyde, hydrogen peroxide, iodophors, ortho-phthalaldehyde (OPA), peracetic acid, phenolics, quaternary ammonium compounds (Quats), and chlorine. Most of the chemical disinfectants used in Minnesota facilities must be managed as hazardous waste (for unused or expired quantities), and disposal options are discussed in the healthcare hazardous waste section of this website. Note that some disinfection techniques are more environmentally preferable than others, and best practices are discussed below. - Sterilization
Sterilization virtually eliminates or destroys bacteria and viruses. In addition to disinfection, the CDC guidelines also provide guidance on sterilization. The sterilization methods discussed in the document include steam sterilization, ethylene oxide (EtO), hydrogen peroxide gas plasma, and liquid peracetic acid. Note that some sterilization techniques are more environmentally preferable than others, and best practices are discussed below.
What are Some Environmentally Preferred Techniques for General Surface Cleaning?
There are many options for a healthcare facility to “green” its general surface cleaning program.

Commit to using cleaning products that are certified as GreenSeal or EcoLogo. Green cleaners are generally defined as those that have less environmental and health impacts. Both Green Seal and EcoLogo have developed stringent criteria in order to authenticate what can be labeled as a “green” cleaner and both are well respected certification organizations. Green cleaners can be used in many places within a healthcare facility. This may include products such as degreasers, glass cleaners, carpet cleaner, bathroom cleaners, and multi-surface cleaners.
Recommendation #2:
Commit to using automatic dilution systems for your cleaning products. Systems such as 3M’s Twist n’ Fill use a dispensing technology that automatically measures the chemical-per-water ratio for your cleaning products and dispenses them safely and accurately. Automatic dilution systems are better options environmentally because they reduce over-use; you only use what is needed. Finally, because the chemicals come in a concentrated quantity and are intended to be dispensed into reusable spray bottles and buckets, automatic dilution systems also reduce solid waste.
Recommendation #3:
Give careful consideration to purchasing in bulk, especially in smaller facilities or for products that are used more slowly than others. When facilities find themselves with outdated cleaning products, many then require disposal in a hazardous waste stream. To prevent this expensive waste, you should evaluate the ordering process your housekeeping, supply chain, and maintenance staff use for chemicals. “Buy in bulk” discounts are no help if a product expires. If you find that you have more on the shelf than you can use before expiration, try calling others in your healthcare facility network to share your product before it expires, or try listing it on the Minnesota Materials Exchange.
Recommendation #4:
Commit to becoming a fragrance-free facility. Fragrances, however well intended, can trigger allergy and asthma attacks and cause headaches or irritation to the eyes, nose, and throat. Researchers at the University of Washington analyzed just 25 commonly used fragranced products and found they contained more than 133 volatile organic compounds (VOCs), impacting indoor air quality for everyone. It is good policy to eliminate fragrance wherever you can in products like cleaners, air fresheners, detergents, and soaps.
Use an integrated pest management (IPM) system. According to the Environmental Protection Agency’s page on Pesticides and IPM, “IPM is not a single pest control method but, rather, a series of pest management evaluations, decisions and controls. In practicing IPM, those who are aware of the potential for pest infestation follow a four-tiered approach.” The four steps are 1) setting action thresholds, 2) monitoring and identifying pests, 3) prevention, and 4) control. The idea here is that common sense should be used when applying pesticides (hazardous chemicals) for pest elimination. Most major pest control companies offer IPM and many major institutions practice it; IPM may provide an environmentally preferable opportunity without compromising the quality of your system.
What are Some Environmentally Preferred Techniques for Disinfection?
Though there are no “green” disinfectants currently registered with the EPA, there are many options for a healthcare facility to “green” its overall disinfection program.
Recommendation #1:
Switch from glutaraldehyde to an ortho-phthalaldehyde (OPA) solution. Glutaraldehyde is a respiratory irritant and sensitizer and may pose problems at the wastewater treatment plant. OPA (the most common being Cidex) has replaced glutaraldehyde in many healthcare facilities because it is not known to irritate the eyes and nasal passages, does not require activation or exposure monitoring, and has a 12-minute high-level disinfection claim. Take note that unused or expired glutaraldehyde and OPA must be treated as healthcare hazardous waste. Used OPA can generally be rinsed down the drain; however, facilities are encouraged to neutralize their used OPA solutions before discharging to the sewer. Several products are on the market that effectively neutralize OPA solutions.
Recommendation #2:
Understand the difference between low-level, intermediate-level, and high-level disinfectants. Use the lowest level that is recommended for each particular area and properly manage any waste associated with your disinfectants. Most of the chemical disinfectants used in Minnesota facilities must be managed as hazardous waste (for unused or expired quantities); disposal options are discussed in the healthcare hazardous waste section of this website.
Recommendation #3:
Provide adequate training and personal protective equipment for all staff that are required to administer disinfectants as part of their duties. Disinfectants are usually more dangerous for a worker than general cleaning products. As discussed in the Employee-Right-to-Know section of this webpage, a safe and healthful work environment includes preventing workers from potentially dangerous fumes, splashes, spills, or direct skin contact. Be especially diligent around women who are pregnant or are of child-bearing age..
Recommendation #4:
As with general surface cleaning products, give careful consideration to purchasing in bulk, especially in smaller facilities or for products that are used more slowly than others. When facilities find themselves with outdated cleaning products, many then require disposal in a hazardous waste stream. To prevent this expensive waste, you should evaluate the ordering process your housekeeping, supply chain, and maintenance staff use for chemicals. “Buy in bulk” discounts are no help if a product expires. If you find that you have more on the shelf than you can use before expiration, try calling others in your healthcare facility network to share your product before it goes bad, or try listing it on the Minnesota Materials Exchange.
Recommendation #5:
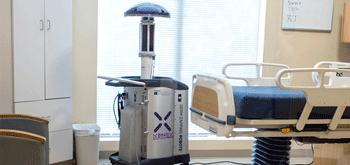
Research whether a portable machine such as Xenex (pulse xenon UV disinfection) would be a viable option for your facility. The Xenex machine uses high energy ultraviolet light (in a spectrum called UV-C) to destroy harmful bacteria and viruses without chemicals. This technology is endorsed by Practice Greenhealth as well as the Association for Professionals in Infection Control and Epidemiology (APIC). Xenex machines have become a popular disinfection tool in the fight against hospital acquired infections (HAIs). Visit the Xenex website for more details.
What are Some Environmentally Preferred Techniques for Sterilization?
Sterilization is one of the most difficult processes to “green”; here are a few recommendations.
Recommendation #1:
Phase out any use of ethlyne oxide (EtO). Ethlyne oxide is a colorless, flammable, toxic, highly reactive compound that is used to sterilize moisture-sensitive or heat-sensitive surgical instruments. EtO has proven to be an effective sterilization method; however, it is also a carcinogen and can cause blindness, liver and kidney damage, and severe burns to skin. Due to these risks, the EtO sterilization process requires the use of additional ventilation, personal protective equipment, and pollution control equipment to treat residuals. There are very few medical instruments that require EtO sterilization these days, so it would be worth researching whether you can switch to a less harmful sterilization process, such as steam or liquid paracetic acid.
Recommendation #2:
Switch to reusable sterilization hard-cases instead of using blue wrap. Sterilization containers are stainless steel boxes in which surgical instruments are placed before being sterilized. They can be reused hundreds of times and can be substituted for blue wrap in many applications. The use of hard cases minimizes the quantity of sterilization blue wrap and tape used and disposed of, thus greatly reducing waste and costs. MnTAP has done two case studies on the use of sterilization hard cases, both found in the case studies section below.
Recommendation #3:
Commit to using lead-free indicator tape on your blue wrap. Though it is environmentally preferable to use sterilization hard cases (#2 above), there are times in healthcare facilities when blue wrap (and its associated indicator tape) cannot be avoided. 3M now sells a lead-free indicator tape called 3M Comply. It works well and has been widely adopted by healthcare facilities. The best part about using lead-free indicator tape is that you do not need to dispose of your used tape in an expensive hazardous waste stream.

Recycle your blue wrap. Blue wrap is used to wrap surgical instruments and trays prior to steam sterilization. It is a #5 polypropylene plastic and is easily recyclable when kept separate from other materials. Minnesota Waste Wise Foundation, part of the Minnesota Chamber of Commerce, offers a blue wrap recycling program to many geographic areas within Minnesota. Though it is preferable to use sterilization hard cases (#2 above), there are times in healthcare facilities when blue wrap cannot be avoided. Visit the Minnesota Waste Wise Foundation website for more information about how to join the blue wrap recycling program.
Recommendation #5:
Finally, ensure that your steam sterilizers (sometimes called autoclaves) are running at optimum capacity and efficiency. Steam can be an energy-intensive sterilization method, so to reduce energy and water consumption, try to run loads only when the machine is full. In addition, some sterilizer brands, such as Steris, offer modification kits to convert water ejectors with vacuum pumps, reducing water usage by about 25% in these machines.
Case Studies, Resources, and Links
There are many interpretations of what may be acceptable best practice with regard to cleaning, disinfection, and sterilization. Provided below are some resources that may help you explore each of these topics in greater depth so that you decide if any of the above recommendations would be acceptable to you and your Infection Control Practitioner.
Resource #1:
Links cited in the document above (for those who may be using a print version of this information).
- Latest CDC information about healthcare associated infections (HAIs)
- Free CDC training videos on how to prevent and protect patients from HAIs
- OSHA information on Infectious Diseases and how to protect healthcare workers
- Personal Protective Equipment (PPE) and how to complete a workplace “hazard assessment”
- 2008 CDC Guidelines for cleaning, disinfection, and sterilization
- EPA registered disinfectants
Resource #2:
Healthcare Without Harm has a wealth of information related to chemicals and healthcare, such as:
- Healthcare Without Harm Main Website
- Green cleaning in healthcare (using a case study of five hospitals)
- Risks to asthma posed by indoor health care environments
- Ten reasons to eliminate glutaraldehyde
- Controlling pests without harmful pesticides
- Cleaning in healthcare facilities
Resource #3:
Practice Greenhealth is a nonprofit membership organization; their mission is to empower its healthcare industry members to increase their efficiencies and environmental stewardship while improving patient safety and care through tools, best practices and knowledge. Many resources are provided to members only; however, you can still find free materials on their website, such as:
- Practice Greenhealth Main Website
- Chemicals (such as chemical policy development, green cleaning, disinfectants, sterilants)
- Environmentally Preferable Purchasing
- Greenhealth academy webinars on a wide variety of topics (nominal cost for nonmembers)
Resource #4:
The Alliance for the Prudent Use of Antibiotics (APUA) is an organization dedicated to reducing the use of antibiotics. Developed at Tufts University, this group strives to “guide policy makers, provide organizations, and other stakeholders seeking to improve antimicrobial supply, use, and management decisions.”
- Alliance for the Prudent Use of Antibiotics (APUA) Main Website
- Information for practitioners on hygiene and infection control
Resource #5:
As a healthcare practitioner, you can be an advocate for chemical reform. Joining the cause as an individual healthcare practitioner or as an entire organization is a great place to start. The three organizations below offer a wealth of information on chemical reform as well as many opportunities for advocacy, volunteerism, and other ways to get involved.
- Safer Chemicals Healthy Families (they also have a healthcare working group)
- Physicians for Social Responsibility
Resource #6:
MnTAP intern projects on infection prevention and green cleaning.